Sclerotherapy Technique for Telangiectasias
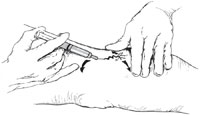
When performing sclerotherapy, the skin should be held taut to facilitate cannulating the vessel. This can be achieved by stretching the skin in opposite directions perpendicular to the vessel with one hand. Then, with the opposite hand that is holding the syringe, the fifth finger is used to stretch the skin in a third direction away from the vessel. These three tension points ensure that the skin is taut and ready for injection (Fig. 8.6). The ultimate goal is to enter the vessel and inject the sclerosant within, and not outside, the vessel wall [2]. A 30-gauge needle will usually yield the desired results, with maximum comfort for the patient as well.However, some phlebologists recommend using either a 32- or 33-gauge stainless steel needle for the intravascular injection of smaller telangiectatic vessels, even though these needles are nondisposable, require sterilization, and dull and bend easily. Also recommended is a 3-ml syringe filled with 2 ml of sclerosant, as this allows for slow, low-pressure injection of the sclerosing solution and avoids “blow-out” of the vessel and extravasation. Each injection should take approximately 5–15 s [1]. The 3-ml syringe is also an ideal size and can be manipulated easily (Table 8.7) [2]. I prefer to aspirate enough air to occupy the needle hub prior to injecting. The air that enters the vessel displaces the blood and assures that the needle is in the vein. If a diffuse urticarial- like blanching is observed, the needle is not in the lumen (the air has entered the surrounding tissue). Additionally, as the air pushes the blood through the vessel, the sclerosant makes undiluted contact with the intima, maximizing irritation.Missing the lumen is probably due to the needle being under and not within the vessel. Since most telangiectatic leg veins are located in the superficial dermis of the skin, I recommend placing the needle flat against the skin and penetrating the skin almost parallel to the surface. To ensure depth of penetration and that the vessel is not exceeded, the needle should be bent approximately 45° with the bevel up (Fig. 8.7) [2]. Injecting the vessel with the bevel up lessens the chance of transection.With proper technique and magnification, visualization of the bevel/tip of the needle through the skin and into the vessel is possible to ensure correct placement within the vessel lumen. Further advancement is not required or recommended. Whether sclerotherapy should proceed proximally to distally (the French school), distal to proximal (the Swiss school), or random- site injection, is acceptable and under ongoing discussion. Injecting the most proximal “feeder” vessel in a telangiectatic cluster is preferred. I also advise injecting the “straightest” and largest vessel within the cluster, no matter the direction of orientation, to avoid vascular transection. Edema (urticarial) and erythema become apparent in 2–30 s postinjection and may last 30 min to several hours. The patient may also complain of muscle cramps in the calf or thigh with hypertonic saline and hypertonic glucose/saline injections. This usually lasts less than 3 min, and the patient should be forewarned. Gentle massaging may help with cramping. A bleb at the site of the needle may appear during injection. Removal of the needle and application of digital massage should be performed immediately. I prefer to inject a generous amount of normal saline or 1% lidocaine if this occurs to reduce the pain and help dilute the sclerosant in the tissue. These small infiltrates may leave small brown macules, which usually disappear in 3–12 weeks. It is important to watch the needle site while injecting, rather than the course of the sclerosing solution through the vessel, and to avoid pushing the injecting hand forward while pushing the plunger. If the injection site and needle placement are carefully monitored, then extravasation can be limited. Repeat treatment on persistent vessels can be performed as early as 3 weeks after the previous treatment. Larger-diameter vessels (greater than or equal to 2 mm) may thrombose. This is easily recognized when the patient returns for follow-up and may be apparent as early as 1 week postsclerotherapy. The vessel appears bluish-purple and does not blanch under pressure. Treatment consists of making a small “stab” incision over the vessel with a number 11 blade and milking out the dark, syrupy blood. The wound is covered with a topical antibiotic ointment and bandage and usually heals well. Maximum recommended dosages of sclerosants vary with the different types and concentrations (Tables 8.8 and 8.9). Many phlebologists recommend a treatment session time of approximately 15–30 min and not more than 12 cc of sclerosant per session [10]. Sclerotherapy requires great concentration and a steady hand. Clinician fatigue greatly reduces efficacy.
Compression should be applied to the injected site immediately postinjection. Massaging the injected vein(s) immediately after withdrawing the needle, using firm pressure and “milking” the sclerosant toward the smallest telangiectatic branches, provides immediate compression and decreases the chance of sclerosant and venous blood reflux from the puncture site and into the surrounding tissue. Massaging may also limit bruising and minimize stinging and burning. Adequate compression following each sclerotherapy session is essential for optimization of both short- and longterm treatment results. Direct contact of the sclerosed endothelium via compression results in more effective fibrosis and allows for the use of lower concentrations of sclerosant [11, 12, 13]. Compression also reduces the extent of thrombus formation, which in turn decreases the incidence of vessel recanalization. Postsclerosis hyperpigmentation and telangiectatic matting (TM) have also been shown to be reduced with the use of postsclerotherapy compression [12, 14]. Compression following treatment also improves efficacy of the calf-muscle pump and aids in more rapid dilution of the sclerosant from the deep venous system, thereby reducing the risk of deep venous thrombosis [2, 11, 12]. Patients who undergo sclerotherapy for uncomplicated telangiectasias usually can wear lighter-weight, graduated compression stockings (class I, 20–30 mmHg).These garments are applied at the end of the treatment session,with the treated leg(s) elevated approximately 45° above the horizontal. Additionally, postsclerotherapy cotton balls or rolls or foam pads are applied over the larger treated vessels and applied firmly in place with adequate pressure with a wide elastic bandage prior to application of the graduated compression garment (Table 8.7). Intravascular clots and phlebitis often occur when larger vessels are not additionally compressed with padding. Some phlebologists advocate removal of the compression garment 6 weeks after sclerotherapy, while others advocate wearing compression for no more than 8 h postsclerotherapy [10, 11, 12, 13]. Those who advocate 8 h of postsclerotherapy compression for telangiectasias feel the final outcome is no different than with patients who wear compression for 6 weeks [14]. The general recommended duration for wearing compression stockings varies from 3 days to 6 months, depending on– among other things–the diameter of the vessel(s). Studies show the maximum benefits of compression garments, no matter how long they were worn,were seen between 3–6 months following treatment [12]. The most improvement was seen in patients who wore the compression stockings for 6 months. However, some improvement can be seen in patients who wear the compression stockings for only a few days [12]. Some phlebologists give the patient the option of wearing graduated compression stockings for a period of 1–3 weeks, after expressing to the patient that optimization of treatment is reached with a longer duration of compression. In general, small telangiectasias less than 1mm in diameter may not require any postsclerotherapy compression [6]. After completion of the sclerotherapy session, the patient should walk for approximately 10–30 min immediately following the procedure. The patient should maintain normal day and nighttime activities, including at least a 1 h walk per day for 1 week. Hot showers or baths and strenuous physical activity (aerobics, weight lifting, squatting, etc.) should be avoided for the first week after treatment. Sclerotherapy is considered the standard treatment for intracutaneous varicose veins (spider, telangiectatic, and reticular veins), with an 80–90% improvement rate [15]. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
© 2025 Skin Disease & Care | All Rights Reserved.